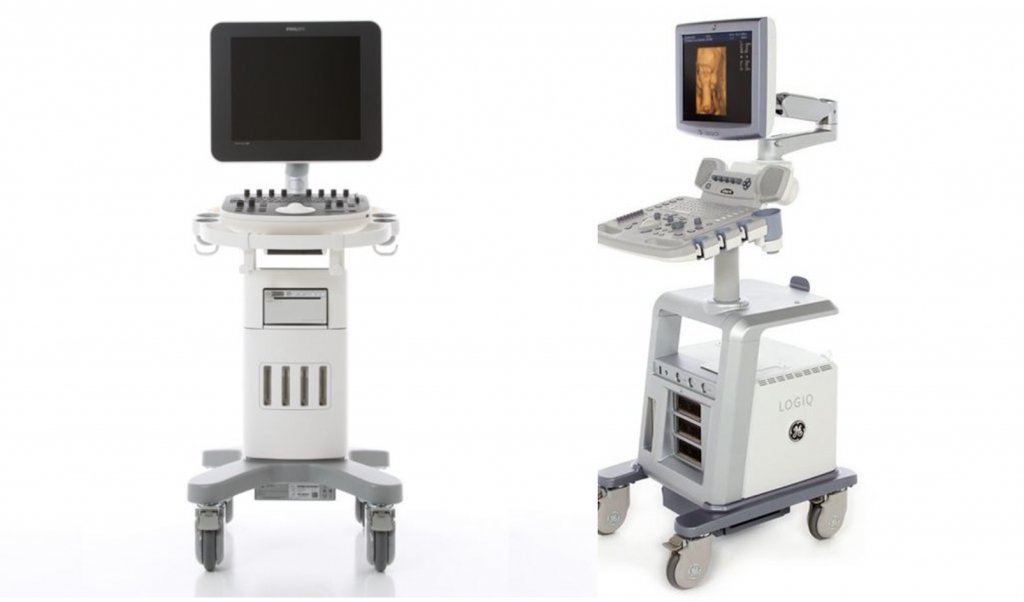
GE Logiq P5 or Philips ClearVue ultrasound machines are both great shared-service machines, catering to applications such as – Abdominal, OB/GYN, Breast, Small Parts, Musculoskeletal, Vascular, Venous, Urology, TCD, Cardiac, General Imaging and Pediatrics. Both Philips and GE ultrasound machines are popular and have their own fan following. Although, Philips ClearVue 350 is the entry level model, it is ClearVue 550 that is generally in competition with GE Logiq P5 in the market.
How does GE Logiq P5 compare with ClearVue 350 or 550 ultrasound machines?
GE Logiq P5 was launched in 2006 and has been going strong for more than a decade after launch. Philips ClearVue 350 and ClearVue 550 were launched in 2012 (replacing the HD Series) and are almost a generation ahead. This generation gap is clearly visible in appearance and hardware itself.
Hardware Configurations
While all three machines are similar in most hardware features, some of the key differences are:
- Philips ClearVue 350 and 550 are both newer than GE Logiq P5 and
- have larger monitor size 17″ vs 15″, ClearVue 550 may have an option of 19” as well.
- higher monitor resolution – 1280*1024 vs 1024*768
- are light-weight 52 Kg vs 75 Kg
- While Philips ClearVue 550 has 4 active probe ports, both Logiq P5 and ClearVue 350 have 3 active probe ports.
- ClearVue 550 offers a tilt, swivel and articulating arm monitor, but, Logiq P5 or ClearVue 350 don’t.
- Both ClearVue ultrasound machines have barcode reader unlike Logiq P5.
That’s about hardware. When it comes to applications, features and imaging modes, there are significant differences.
Application, Imaging modes & Features
While Philips ClearVue sonography machines were launched around 2012, Logiq P5 had already had a stable, solid run and gained the reputation of a reliable performer since 2006. In 2011, GE Logiq P5 got significant upgrades as the Logiq P5 premium BT11 version; the model currently running and largely compared with ClearVue 350 or 550.
GE practically loaded up Logiq P5 Premium with plethora of features and probes, making it formidable. The result was that GE Logiq P5 supported deeper, specialized applications in most areas including Cardiac, Pediatrics, ObGyn, General imaging, Urology etc. The Philips ClearVue 550 is similar to the Logiq P5 in price but has a much more general focus as one would expect from a shared service ultrasound machine. Some of the key differences in features are:
- ObGyn – Unlike Logiq P5, ClearVue 350 & 550 do not offer 4D as base option (but upgradeable) very much popular in high-end Obstetrics area. Logiq P5 also supports Freehand 3D and Auto Follicle 2D measurement. On the other hand, both Philips ClearVue 350 & 550 offer Auto NT measurement as well as Fetal Echo (as an option) – not offered by Logiq P5.
- Cardiac – GE Logiq P5 offers several advanced features for cardiac investigations, that Philips ClearVue systems do not, such as – Tissue Velocity Imaging (TVI), Stress Echo, Auto IMT, Automated LH Measurement, Automated Function Imaging(AFI), Cardiac Motion Quantification(CMQ), Auto EF (Ejection Fraction) and even pediatric/ neonatal echocardiography.
- Urology/ General imaging – GE Logiq P5 supports Elastography, B-Flow, Contrast imaging – General / Cardiac and Panoramic Imaging, resulting in capabilities to do more advanced investigations of abdominal and musculo-skeletal (MSK) area. ClearVue 550 does offer panoramic imaging, but ClearVue 350 does not.
On the whole, we can say while Philips ClearVue has a clear focus on general applications in all common areas and GE Logiq P5 offers more specialization in some of the areas.
Imaging Quality
GE Logiq P5 and Philips ClearVue 550 have all the latest imaging technologies like HD-Speckle Reduction Imaging, Spatial Compounding, Tissue Harmonic Imaging and Auto image optimization for both B and Doppler modes. Although upgradeable for others, ClearVue 350 supports only Spatial Compounding by default.
Imaging technology wise both GE Logiq P5 and ClearVue 550 may tick-off all the same boxes, but quality of ClearVue 550 imaging is superior. Philips ClearVue ultrasound machines deploy Active Array and Broadband beamforming for superb image quality; something comparable to the next generation GE Logiq P7 & P9 (Read more about GE Logiq P series).
GE Logiq P5 vs Philips ClearVue – Probe selection
GE Logiq P5 has extremely extensive range of compatible probes (Almost 29 compatible probes) to cater to equally extensive range of features and applications. Philips ClearVue systems come may be with a quarter of the number of probes. However, all the probes on the Philips ClearVue 550 have a wide frequency range with very good image quality. This means you can do more with each probe and save money because you don’t have to purchase additional probes to cover all the frequencies needed.
Maintenance & Support
Apart from its tremendous range of features, Logiq P5 premium is also extremely popular especially in the refurbished ultrasound machine market, as large quantities of spare parts and probes are easily available at affordable costs. This makes maintenance and support relatively cheaper and easily accessible from third party service outfits. Cost conscious Small and Medium hospitals and diagnostic centres in India prefer GE Logiq P5 for these commercial reasons too.
GE Logiq P5 or Philips ClearVue ultrasound machines – Its your choice!
—————————————-
PrimedeQ is an eMarketplace for medical equipment. We offer all types of imaging equipment including used / refurbished ultrasound machines from GE, Philips, Seimens, Samsung, Toshiba and others. We also assist in ultrasound machine repair and probe repair & maintenance services at www.Primedeq.com/services. Contact us at +918971223957 or +917019759765 for all your medical equipment related needs.
https://in.linkedin.com/in/shanthi-mathur-ab07838
